OUTRACE Studies Resource Library
This website provides information and resources on the OUTRACE studies, which are evaluating the T cell engager, CLN-978. CLN-978 is an investigational therapeutic approach that has the potential to offer a convenient, off-the-shelf, subcutaneously delivered therapeutic option for people living with autoimmune diseases.
OUTRACE Studies
CLN-978 is being evaluated for use in the following conditions. Patients can navigate to clinicaltrials.gov or euclinicaltrials.eu by clicking the links below. Clinical trial sites may access their respective study portals by clicking on the blue access button(s) in each study card and entering in their username and password details.
Rheumatoid Arthritis (RA)
CLN-978 is currently being studied in a Phase 1 clinical trial in patients with treatment-refractory RA.
Access RA MaterialsSjögren's Disease
CLN-978 is currently being studied in a global Phase 1b clinical trial in patients with moderate to severe Sjögren's Disease.
Access Sjögren's MaterialsSystemic Lupus Erythematosus (SLE)
CLN-978 is currently being studied in a global Phase 1b clinical trial in patients with moderate to severe SLE.
Access SLE MaterialsINTRODUCTION TO TCEs AND CLN-978
ABOUT CLN-978
An overactive immune response, driven by B cells and/or T cells, may lead to the development of autoimmune diseases.
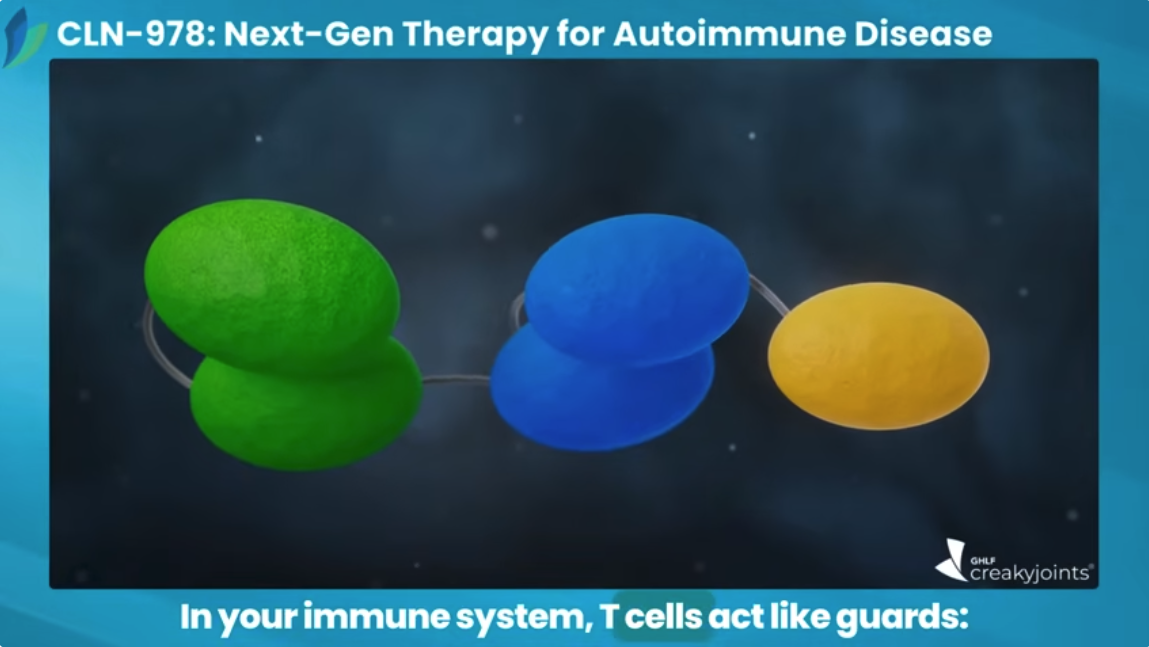
CLN-978 is a subcutaneously* administered CD19-directed T cell engager. T cell engagers are an emerging therapeutic approach in autoimmune diseases, with the potential to modulate immune responses in ways that may enable durable disease control and advance current treatment strategies:
- T cell engagers can harness the immune system in autoimmune diseases to eliminate disease-causing cells.
- T cell engagers like CLN-978 are bispecific antibodies designed to guide the immune system’s T cells to find and eliminate harmful cells, such as autoreactive cells that carry specific markers on their surface.
- CLN-978 works by linking T cells and B cells together by binding to CD3 on T cells and CD19 on B cells.
- Once the T cell is linked with the B cell, the T cell then destroys the targeted harmful cell.
We are currently evaluating CLN-978 in people living with Systemic lupus erythematosus, Rheumatoid arthritis, and Sjögren’s disease. CLN-978 is investigational and has not been approved by any health authority.
*A subcutaneous injection is an injection under the skin.